Calculate Kp for the Reaction 2nocl 2no Cl2
I 2NOCl g ƒ 2NO g Cl2 g. Question 74 Write the expression for the equilibrium constant Kc for each of the following reactions.
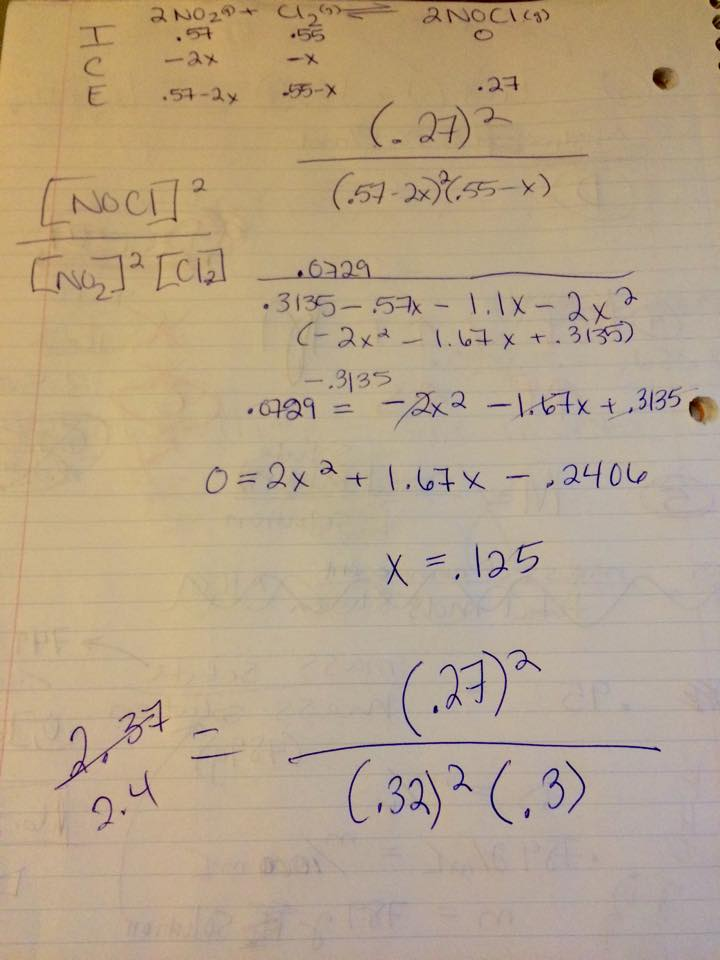
Solved Consider The Reaction Between No And Cl2 To Form Chegg Com
COg Cl2g arrow COCl2g A reaction mixture initially contains a CO concentration of 01550 M and.
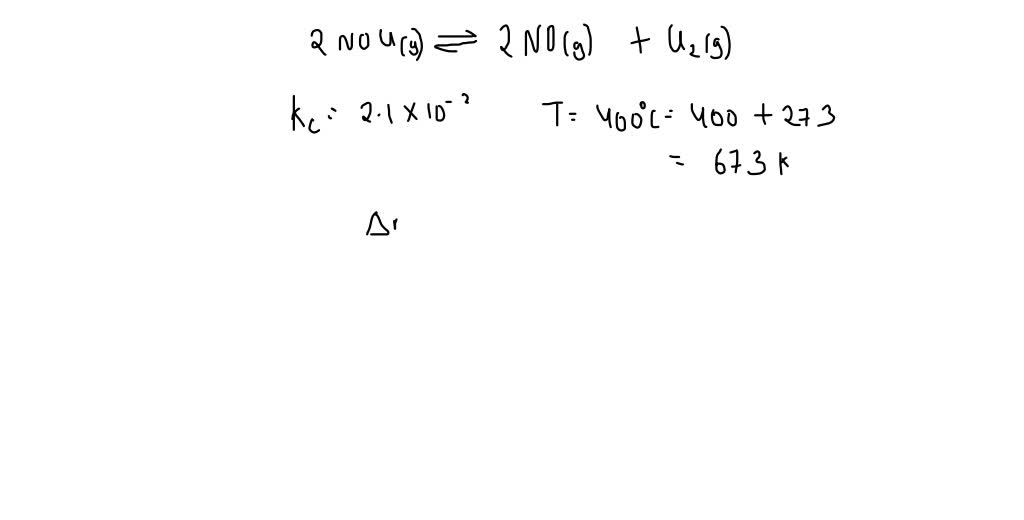
. Q K so the. For the following reaction Kc 255 at 1000 K. Question 73 At a certain temperature and total pressure of 105Pa iodine vapour contains 40 by volume of I atoms I2 g ƒ 2Ig Calculate Kp for the equilibrium.
At a given point the partial pressures of the gases are PN2PO2 0660 atm and PNO 00272atm. For the equilibrium N2gO2 2NOg Kp 00017 at 2300 K. Q K so the reaction will continue to make more products.
Which statment below is true. Q K so the reaction will consume products to make more reactants.

The Value Of Kp For The Reaction At 500k 2nocl G 2no G Cl2 G Is 1 8 10 2bar 1 Calculate Kc For The Reaction

The Value Of Kp For The Reaction At 500k 2nocl G 2no G Cl2 G Is 1 8 10 2bar 1 Calculate Kc For The Reaction

For The Equilibrium 2nocl G Harr 2no G Cl 2 G The Value Of The Equilibrium Constant K Youtube

For The Equilibrium 2nocl G 2no G Cl2 G The Value Of The Equilibrium Constant Kc Is 3 75 Chemistry Equilibrium 13145325 Meritnation Com
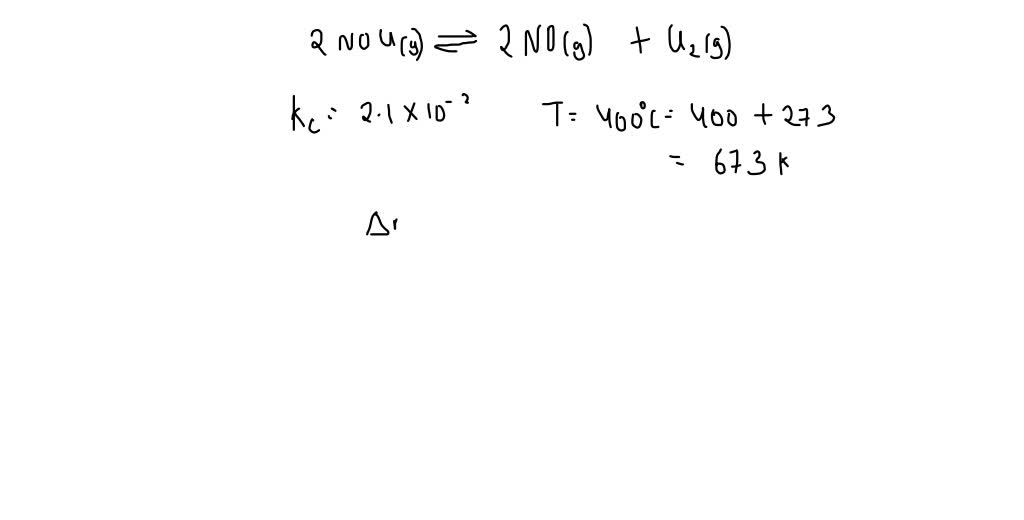
Solved Q Calculate Kp For The Reaction 2nocl G A 2no G Cl2 G At 400a C If Kc At 400a C For This Reaction Is 2 1 X 10a 2 A 2 1 X 10a 2 B 1 7 X 10a 3
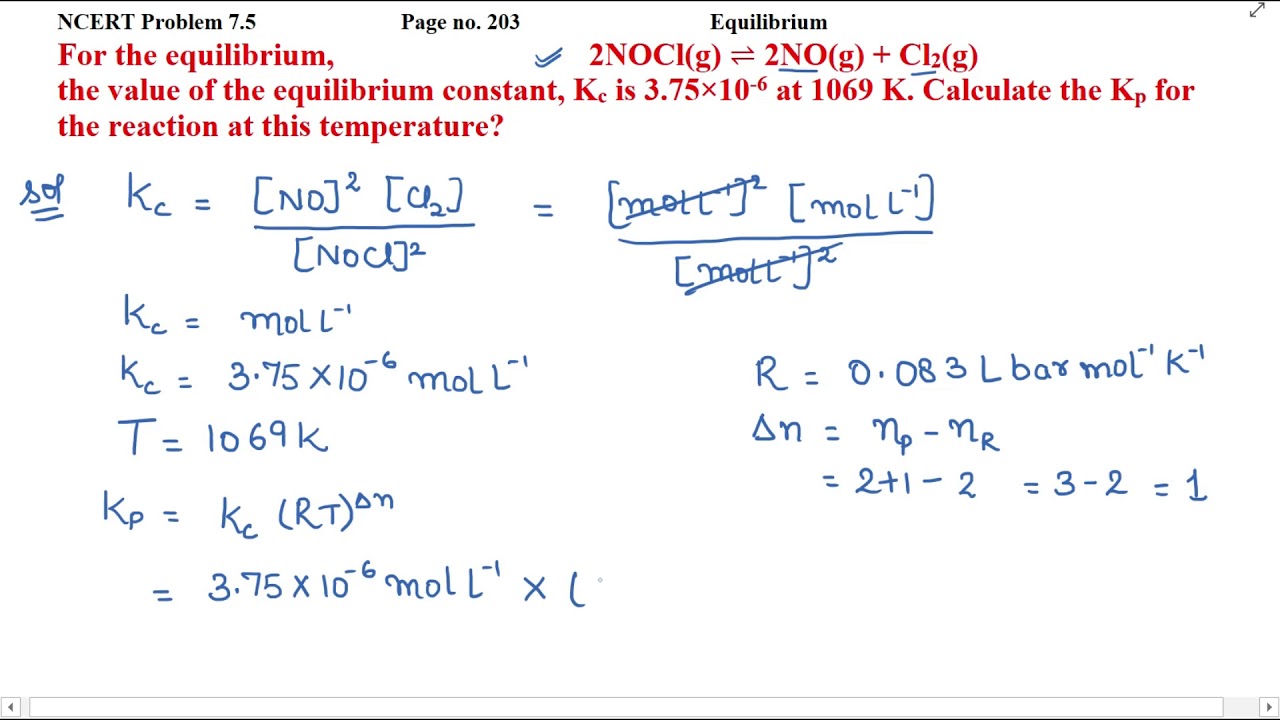
For The Equilibrium 2nocl G 2no G Cl2 G The Value Of The Youtube

Solved 2 Calculate Kp For The Reaction Below At 400 C If Kc Chegg Com

For The Reaction 2nocl G Harr 2no G Cl 2 G Calculate The Standard Equilibrium Constant A Youtube

6 For The Equilibrium 2nocl G 2no G Cl2 G The Value Of The Equilibrium Constant Kc Chemistry Equilibrium 16487787 Meritnation Com
No comments for "Calculate Kp for the Reaction 2nocl 2no Cl2"
Post a Comment